Recombinant antibody technology has been instrumental in overcoming many challenges associated with traditional hybridoma platforms, enabling the development of a new class of biologic drugs. This technology continues to play a crucial role in drug development today, particularly in the discovery and enhancement of antibody-based therapeutics and novel modalities.
The Evolution of Recombinant Antibody Technology
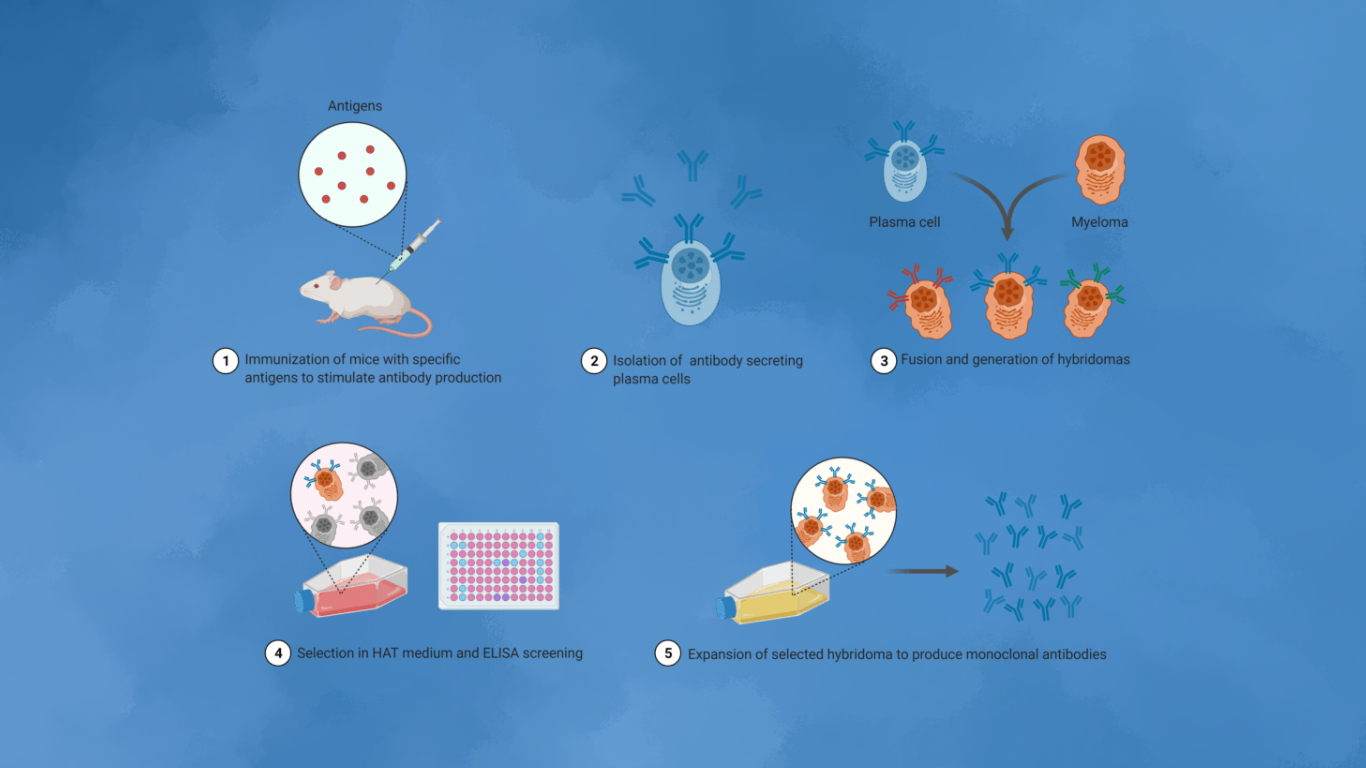
Early Developments and Hybridoma Limitations
Monoclonal antibody (mAb) therapies initially relied heavily on the hybridoma platform, which involves producing the target antigen or immunogen and utilizing animal immunizations. Despite its pioneering role, hybridoma technology presents ethical concerns, issues with antibody heterogeneity, batch-to-batch variability, loss of antibody productivity, and significant limitations concerning cost and scalability. Moreover, the hybridoma process is notably time-consuming, often taking several months from initial immunization to the establishment of specific hybridoma clones and the resulting mAb production.
Advantages of Recombinant Antibody Technology
Recombinant antibodies, generated through host cell lines using recombinant DNA technology, offer a more efficient alternative. This approach significantly reduces the need for animal testing and enables the rapid production of large quantities of products, typically within a few weeks. Recombinant antibodies are based on known DNA sequences, allowing precise replication, controlled production, and consistency. Additional benefits include targeting hybridoma-refractory antigens and enhancing antibody engineering capabilities.
Engineering and Production Benefits
Customization and Efficiency
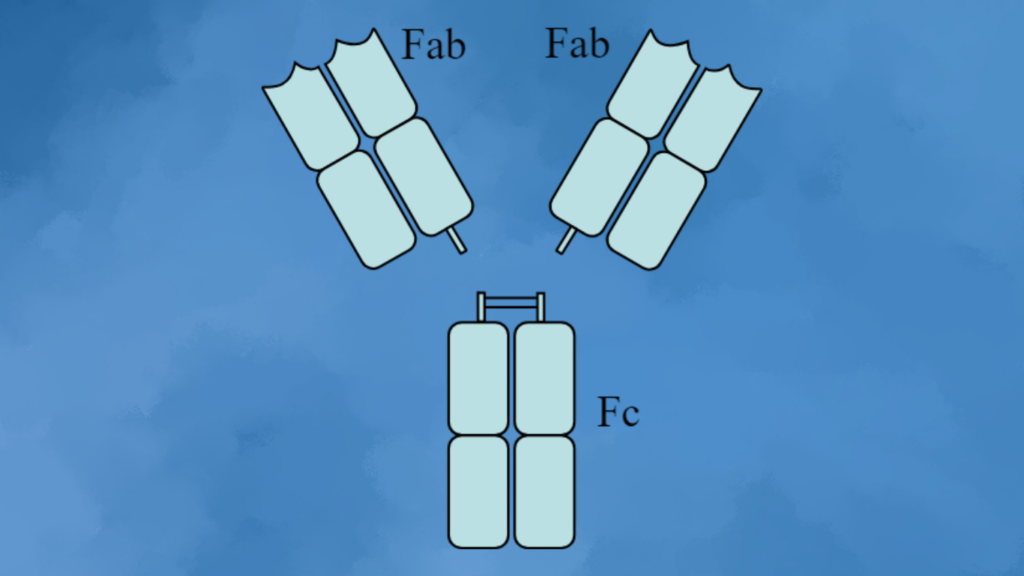
Recombinant antibody engineering offers extensive opportunities for creating unique molecules tailored to treat specific diseases. For instance, modifications can be made to the fragment crystallizable (Fc) region to prevent undesired responses, and variable regions can be engineered for more effective target binding. Antibodies initially discovered in animals can be humanized to reduce potential immunogenicity. With advancements in artificial intelligence (AI), the predictability and efficiency of these engineering processes are further enhanced, allowing high-throughput recombinant antibody production and validation to expedite drug discovery.
Addressing Traditional Antibody Limitations
Traditional mAbs, such as immunoglobulin G (IgG), face limitations in clinical applications due to their size (~150 kDa) and the presence of the Fc region, which can mediate bystander immune activation. Recombinant antibody technology allows the exploration and characterization of various mAbs, including engineered fragments like Fab, scFv, and VHH, and bispecific constructs such as BiTE and Diabody. These fragments retain the targeting specificity of intact mAbs, are better suited for tissue penetration, exhibit reduced immunogenicity, and can bind to traditionally inaccessible epitopes.
Applications and Future Prospects
Biomedical Research and Diagnostics
In biomedical research, antibody fragments with superior tissue penetration capabilities are preferred for assessing molecular and behavioral characteristics. Diagnostic applications benefit from engineered multivalent antibodies with enhanced antigen-binding avidity. Therapeutically, recombinant antibodies are favored for their modifiability, allowing precise targeting and minimizing side effects. They play a pivotal role in developing treatments for various diseases, including cancers, autoimmune diseases, metabolic diseases, and infectious diseases.
Technological Advancements and Future Directions
The field of recombinant antibodies is expected to expand, incorporating novel targets and modifications. As our understanding of genomic and proteomic changes in diseases deepens, new targets for antibody drugs will emerge. The precise design and powerful engineering capabilities of recombinant antibodies make them suitable for medical applications, especially in developing antibodies for novel entities. Innovations in AI and high-throughput production processes will continue to advance the field, making antibody therapies available for previously unaddressed targets.
Case Study: COVID-19 Pandemic Response
The COVID-19 pandemic underscored the importance of recombinant antibody technology. Companies rapidly developed reagents and vaccines using proprietary recombinant platforms, supporting global research efforts. For instance, key SARS-CoV-2 spike protein reagents were produced in record time, demonstrating the technology’s potential for quick response and adaptation to emerging health crises.
Conclusion
Recombinant antibody technology has significantly advanced biopharmaceuticals, offering efficient and ethical alternatives to traditional methods. Its ability to produce highly specific and consistent antibodies rapidly has revolutionized drug discovery and therapeutic applications. As technological advancements continue, recombinant antibodies are poised to play an increasingly critical role in developing targeted, effective treatments for a broad spectrum of diseases.

Subtly charming pop culture geek. Amateur analyst. Freelance tv buff. Coffee lover